COVID vaccines struggle to find their way into the hands of Americans
February 16, 2021
In late 2020, the Pfizer-Biotech vaccine was approved for use by the Food and Drug Administration (FDA), as was the Moderna vaccine. One of the first doses of the Pfizer-Biotech vaccine was administered on Dec. 14, 2020 to a nurse named Sandra Lindsay, at a medical center in Queens, New York. The Biden administration plans to continue administering the vaccines, and as of today over 27 million Americans have received their first dose and over six million Americans have been fully vaccinated.
The Pfizer vaccine must be stored at negative 70 degrees, and the Moderna vaccine must be stored at negative 20 degrees. This can become a problem for those looking to administer the vaccines to the public, as not all facilities can store the vaccines at these extreme temperatures.
The distribution of the vaccine has been separated into three main categories. 1a: healthcare personnel and long-term care facilities, 1b: essential workers (police, firefighters, education sector), and 1c: Adults with high risk medical conditions and adults 65 years and older.
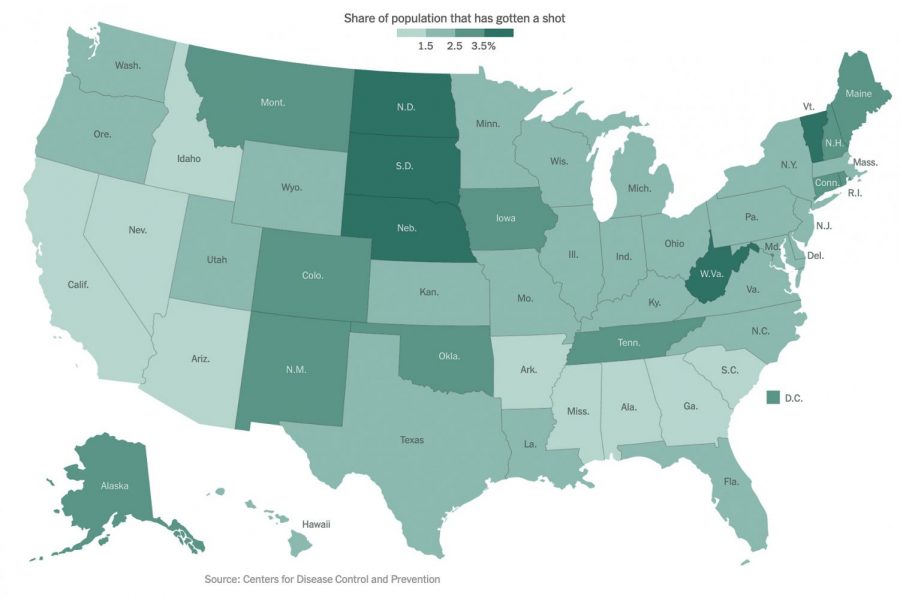
Below is the official CDC distribution plan for more information:
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/COVID-02-Dooling.pdf
There have been several surges in western states due to the recent winter holidays, so many healthcare workers and individuals in the high risk categories are eager to get the vaccine. The days with the highest number of COVID cases have been recorded over the past few weeks, and as schools opened up again after winter breaks, surges of the virus were recorded, resulting in 775,975 new COVID-19 cases in the past seven days alone.